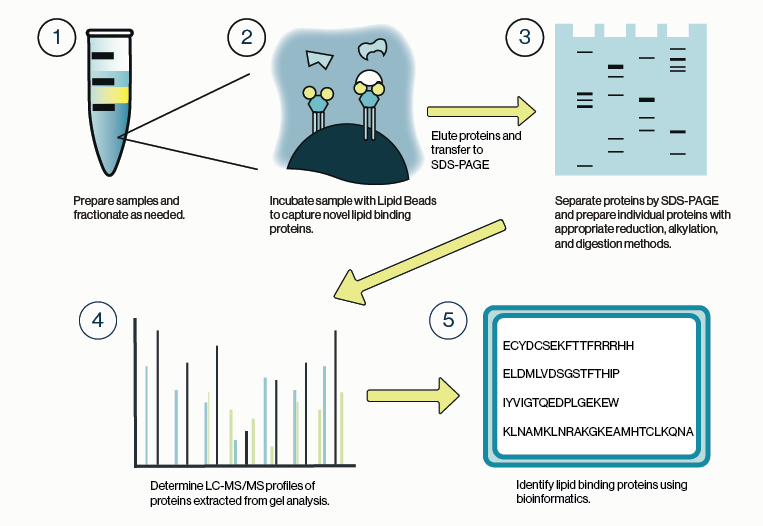
Echelon Biosciences has been making PIP Strips, a supported nitrocellulose membrane spotted with 8 phosphoinositides and 7 other biologically relevant lipids, for almost 20 years. This simple tool is designed as a screen to identify novel interactions between proteins and specific lipids (illustrated above). Images of PIP Strips can be found in publications throughout the lipid field and are known as useful tools in the characterization of new and interesting lipid binding proteins.
The procedure for PIP Strips is very similar to a western blot. The membrane is blocked, incubated with a lipid binding protein of interest followed by primary and secondary antibodies. The PIP Strips are then visualized with chemiluminescence, fluorescence, or with a precipitating TMB (K-TMBp). Although this assay is straightforward, problems do occur. The goal of this blog post is to provide helpful tips and tricks in obtaining quality images and results.
Echelon Biosciences has been making PIP Strips, a supported nitrocellulose membrane spotted with 8 phosphoinositides and 7 other biologically relevant lipids, for almost 20 years. This simple tool is designed as a screen to identify novel interactions between proteins and specific lipids (illustrated above). Images of PIP Strips can be found in publications throughout the lipid field and are known as useful tools in the characterization of new and interesting lipid binding proteins.
The procedure for PIP Strips is very similar to a western blot. The membrane is blocked, incubated with a lipid binding protein of interest followed by primary and secondary antibodies. The PIP Strips are then visualized with chemiluminescence, fluorescence, or with a precipitating TMB (K-TMBp). Although this assay is straightforward, problems do occur. The goal of this blog post is to provide helpful tips and tricks in obtaining quality images and results.
Pro Tip #1: Use Positive Controls
The most common question we receive is “Why did my strip not work?”. Due to this common question, we suggest adding positive controls for your protein of interest and any secondary antibodies used.
To add this control, spot directly onto each PIP Strip 50-200 ng of protein in 0.5-1 μL. Select an area of the membrane that does not interfere with the pre-spotted lipids. Generally, there is space on the top and/or bottom of the PIP Strip for these controls. A second or third positive control should be added for any primary and secondary antibodies used.
Once the positive controls are dry, place the PIP Strip in an incubation box and start the blocking step in the protocol provided. These spots should show a strong signal at the end of your experiment, indicating that the interaction between the protein of interest and the primary and secondary antibodies all performed as expected. This also works as a control for your detection reagent.
If your positive controls show signal but you saw no lipid binding by your protein, that indicates an issue between the lipid and the protein. Since the PIP strip is very stable, if this happens, we suggest exploring potential issues with the protein or trying Pro tip #2: using a control protein.
Pro Tip #2: Use a control protein
With each 10 pack of PIP Strips a free vial of PI(4,5)P2 specific control protein (G-4501) is provided as a + control. This protein control is a well-known lipid binding protein with known stability and binding patterns. Use this control protein to validate your reagents and protocol if no binding is observed with your protein of interest.
We offer an array of lipid binding proteins for you to select from if a different selectivity is needed (see Table 1). The protein you choose will depend on the preferred lipid of your protein. A general protocol on how to use these proteins with PIP Strips can be found on our website.
Table 1: Suggested Control Proteins
| Lipid | Control Protein(s) | Cat# |
| PI(3)P | PI(3)P Grip (p40 PX) | G-0302 |
| PI(4)P | PI(4)P Grip (SidC-3C), Purified Anti-PtdIns(4)P IgM | G-0402, Z-P004 |
| PI(3,4)P2 | Purified Anti-PtdIns(3,4)P2 IgG | Z-P034b |
| PI(4,5)P2 | PI(4,5)P2 Grip (PLC-d1-PH) | G-4501 |
| PI(3,4,5)P3 | PI(3,4,5)P3 Grip (Grp1-PH) | G-3901 |
| Multi-PIP | MultiPIP Grip | G-9901 |
Pro Tip #3: Test different buffers
Buffers and blocking reagents can change the binding pattern of lipid binding proteins and for this reason we recommend testing different buffers and blocking reagents for each protein of interest. As a starting point, there is a list of preferred buffers and recipes on the protocol provided with each order. If you want a visual image of how buffers and reagents can affect your result, look at the last page of the FAQ for PIP Strips.
The pH of your buffer can also play a role in lipid protein interactions. Our PIP Strip protocol is written to use premade buffer tablets but these PBS tablets have some variation in pH (7.1-7.4). The pH variation of the PBS tablets, even in a wash buffer, slightly changed the binding pattern of our proteins. We found that in order to have consistent results, the pH must be controlled (our proteins preferred 7.05-7.25). Always pH your buffers.
Pro Tip # 4: Handle PIP Strips carefully
Caution should be used when handling PIP Strips. Handle them gently with gloves or clean tweezers because oils from your hands can create increased background on the membrane. Small creases and bends in the membrane can increase smears on the strip in the final step. These issues can make it difficult to identify the binding pattern, and honestly, they never look good in a publication.
Pro Tip #5: Titer your protein of interest
For untested proteins we recommend starting with a high concentration of 2 μg/mL. This may cause non-specific binding and increase background, but we find it's easier to start with a binding signal than no signal at all. Once you have binding, the protein concentration can then be titrated down for greater specificity and lower background.
Bonus Pro Tip: Validate your results
PIP Strips are sensitive and powerful tools but caution must be exercised in interpreting results. As discussed in this post, results can be affected by protein concentration, detection reagents, and different blocking and wash buffers. Due to this, we recommend comparing PIP Strip results with other methods and referring to the literature before fully characterizing a lipid binding protein. In addition to PIP Strips, we sell other lipid membrane products, lipid coated beads and plates, and stabilized liposomes (Table 2). All of which, are useful tools in the characterization of proteins and their lipid interactions.
Table 2: Alternative tools for determination of lipid-protein interactions
| Product Group | Product Description |
| Stabilized Liposomes | PolyPIPosomes |
| Lipid coated beads | PIP Beads |
| Lipid coated plates | PIP Plates (H-6200, H-6300) |
| Multiplex lipid beads | Flow PIPs |
Other Lipid Membrane Products we offer
- Membrane Lipid Strips (P-6002), a nitrocellulose membrane with 15 different biologically important lipids found in cell membranes
- Sphingo Strips (S-6000), a nitrocellulose membrane with 15 different and interesting sphinoglipids
- Three types of Lipid Arrays (P-6100, S-6001, P-6003) spotted with a concentration gradient of 8 different lipids.
- Need something different? We make custom strips.
Have other questions? See our FAQ or contact our amazing technical service department.
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences