Immunostaining is a long-standing method that utilizes antibodies to label biological targets in live and fixed cells and tissues. The majority of immunostaining reported in the scientific literature is centered on proteins, but lipid immunostaining is also possible.
General immunostaining of cells or tissue (immunocytochemistry, ICC and immunohistochemistry, IHC) typically requires mounting on glass coverslips or slides. Reagents such as methanol or formaldehyde are then used for chemical fixation. The cells must then be permeabilized using a detergent solution for the antibodies to gain access to the intracellular compartment. These steps can present barriers when using anti-lipid antibodies for immunostaining. Chemical fixation is necessary but may mask or destroy the epitope of the lipid antibody. Additionally, the type and concentration of detergent may disrupt the lipid or membrane to render them undetectable. However, neither of these barriers are insurmountable.
Fixation and Permeabilization
For chemical fixation, formaldehyde (FA) is preferable over methanol as it does not dehydrate cells or tissue. As FA is a crosslinking reagent, epitope masking may still be a concern, but this can be easily addressed by modulating the concentration of FA and the time that it is applied. Concentrations of FA that are 4% (w/v) or less for 10-15 min have been successfully used for immunostaining with lipid antibodies (Echelon, Product Z-P345; Z-P004). Similarly, for frozen tissues and IHC, brief application of 10% neutral buffered formalin (an FA containing fixative) for 2 min was sufficient for downstream staining with a lipid antibody (Echelon, Product Z-P200). Low concentrations of glutaraldehyde can also be added to facilitate staining of specific compartments.
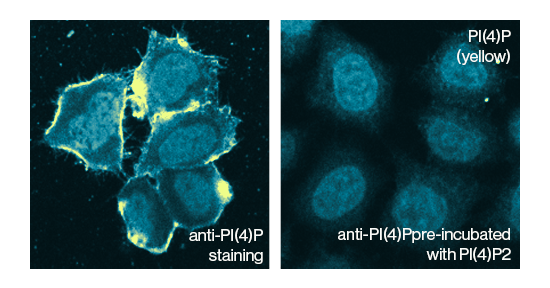
Permeabilization also requires careful consideration. For one, if the target lipid is in the plasma membrane, then permeabilization may not be required at all. For intracellular lipids, it is again advisable to temper both detergent concentration and application time as well as the type of detergent. Triton-X 100 is a common detergent used for immunostaining with concentrations of 0.5% (v/v) or less being suitable for lipid antibodies (Echelon, Product Z-G345). A frequently used detergent alternative is saponin, an amphipathic molecule with surfactant-like characteristics that allow for membrane penetrance. Saponin can be readily used at concentrations similar to those seen with Triton-X 100 (Echelon, Product Z-B045).
Resources and Other Considerations
Professor Gerry Hammond and colleagues have also been instrumental in this area by establishing a series of protocols for staining lipids in various intracellular compartments. These methods further address the main issues associated with lipid immunostaining by systematically analyzing the effects of fixation and permeabilization conditions on binding of PIPn antibodies in various cellular compartments1, 2, 3. Echelon has used this work and other literature to produce a general protocol that we recommend as a starting point for lipid immunostaining.
In summary, staining with lipid-antibodies is entirely feasible if the above-mentioned concerns are addressed during experimental design. Echelon houses a wide selection of lipid-antibodies that have been used to generate beautiful fluorescent images in both ICC and IHC applications. Happy staining!
References
- Hammond GR, Dove SK, Nicol A, Pinxteren JA, Zicha D, Schiavo G. Elimination of plasma membrane phosphatidylinositol (4,5)-bisphosphate is required for exocytosis from mast cells. J Cell Sci. 2006;119(Pt 10):2084-94.
- Hammond GR, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, et al. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science. 2012;337(6095):727-30.
- Hammond GR, Schiavo G, Irvine RF. Immunocytochemical techniques reveal multiple, distinct cellular pools of PtdIns4P and PtdIns(4,5)P(2). Biochem J. 2009;422(1):23-35.Echelon