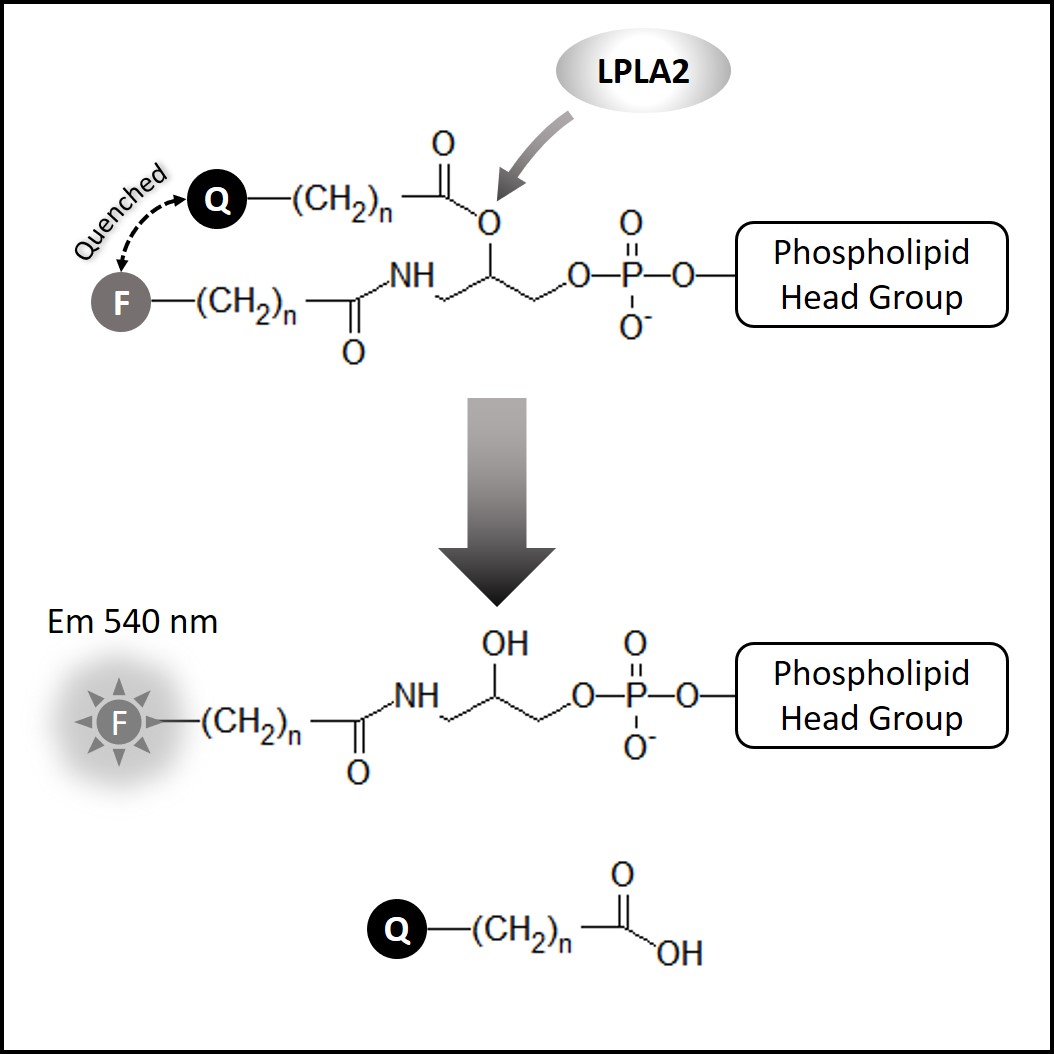
Echelon’s LPLA2 Inhibitor Screen is a high throughput assay designed to screen drugs abilitiy to inhibit LPLA2 activity in vitro, a potential predictor of drug-induced phospholipidosis.
Sample Volume: 1 μL
Sample Number: 128 Samples (in triplicates)/384-well plate
Echelon’s LPLA2 Inhibitor Screen (K-7000I) is designed to assay a drug’s ability to inhibit LPLA2 activity in vitro, and thus potentially predict DIPL. The assay uses a quenched substrate which fluoresces when hydrolyzed by LPLA2. This direct biochemical approach provides a quantitative measurement in a robust and simple-to-use in vitro plate-based assay, providing greatly increased throughput when compared to traditional microscopy methods of tissue cultured cells. The assay has been validated with a group of known PL-inducing and non-PL inducing drugs. The known PL-inducing cationic amphiphilic drug, amiodarone, is included in the assay as a control.
Product Background
Human lysosomal phospholipase A2 (LPLA2, also known as Lp-PLA2 or PLA2G15) is responsible for normal lipid metabolism and it is unique from other known PLA2s in that LPLA2 is only active in an acidic environment (~pH 4.5). Phospholipidosis (PL) is a condition resulting from the excessive accumulation of intracellular phospholipids, causing tissue inflammation and organ damage. PL can manifest in patients taking certain cationic amphiphilic drugs (CADs) such as fluoxetine (Prozac™, Sarafem) and Amiodarone. The FDA has determined that drug-induced phospholipidosis (DIPL) is a serious drug safety issue and evidence is accumulating that DIPL is the result of certain CADs directly inhibiting LPLA2.