α-Synuclein (α-Syn) has long been associated with neurodegeneration due to its presence in abnormal protein aggregates found in the brains of Parkinson’s Disease (PD) patients. Later genetic work identified multiple mutations in the gene that encodes for α-Syn, SNCA, that were associated with familial PD and some sequence variations in SNCA are apparent in sporadic PD. This has led to a large body of research investigating both the function of α-Syn as well as strategies for tuning its expression as potential means of therapy.
α-Syn was initially identified as a synaptic vesicle protein and subsequent biochemical research demonstrated that it was associated with components of the endocytic machinery including endophilin, AP2, and clathrin. Neuronal knockout of α-Syn and its gene homologs slows endocytic recycling of synaptic vesicles and perturbs the kinetics of neurotransmission. This supports the idea that α-Syn interacts with and affects the dynamics of the endocytic machinery in neurons. In the current study, the researchers hypothesized that α-Syn directly affects the assembly of clathrin on the neuronal membrane.
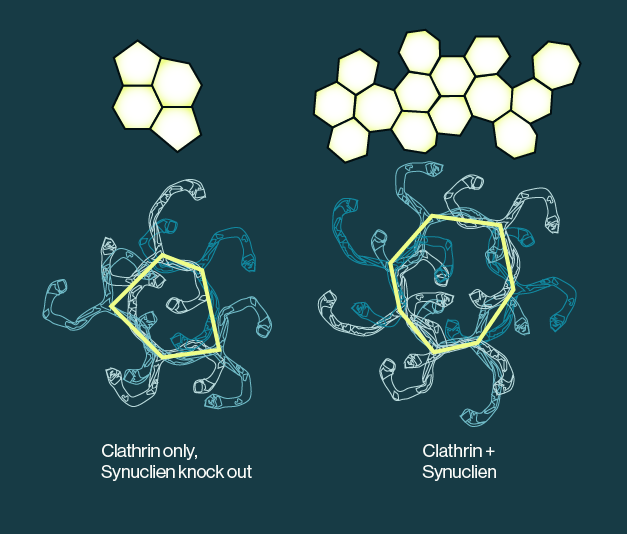
Figure 1: Addition of alpha synuclein affects the size and shape of clathrin lattices on in vitro physiological membranes. The presence of synuclein appears to shift the geometry of clathrin assemblies and act as a primer for clathrin assembly.
Using a combination of recombinant proteins, synthetic lipid membranes, and various imaging techniques, Vargas and colleagues were able to show that α-Syn affects the size of clathrin lattices on physiological membranes. Evidence from fluorescent imaging shows that α-Syn colocalizes with clathrin and the adaptor protein AP180. Association of α-Syn with clathrin lattices on the membrane was also dependent on the presence of the lipid PI(4,5)P2, which has been previously shown to bind α-Syn. Additional electron microscopy imaging revealed that the presence of α-Syn affected the size and curvature of clathrin lattices and suggests that α-Syn may shift from the surface of synaptic vesicles to the synaptic membrane to act as a primer for clathrin assembly. The current data outline a physiological mechanism for loss of some neuronal function over time in PD, independent of the presumed toxic protein aggregates that are associated with the disease. This study also suggests that restoring α-Syn function in patients may be a viable therapeutic strategy.
Read the full article here:
α-Synuclein colocalizes with AP180 and affects the size of clathrin lattices
Journal of Biological Chemistry 299 (9):105091 (2023)
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences