The development of kinase inhibitors has received significant attention, with relatively little emphasis on creating pharmacological kinase activators. This is surprising, as both protein and lipid kinases are known to have positive functions such as tissue regeneration and immune activation. The lipid kinase PI3K is no exception to this gap in research as it has been primarily targeted with inhibitors to address overactivation in cancer and certain immune disorders. While hyperactive PI3K is disease-associated due to its functions in cell growth and proliferation, this does not rule out harnessing its activity therapeutically.
Inhibition of PI3K has been shown to blunt the protective effects of growth factors during tissue repair and neuroprotection. To some extent, this should be expected as PI3K is a crucial signaling hub between several tyrosine kinases and downstream effectors such as AKT and mTORC. However, the strategies that have been pursued for PI3K activation thus far have had narrow specificity and unclear mechanisms of PI3K activation. These include, but are not limited to genetic alteration of PI3K binding proteins and small molecules or peptides. In the current study, Gong and colleagues sought to address this issue by conducting an unbiased screen for PI3K activators in a physiologically relevant context.
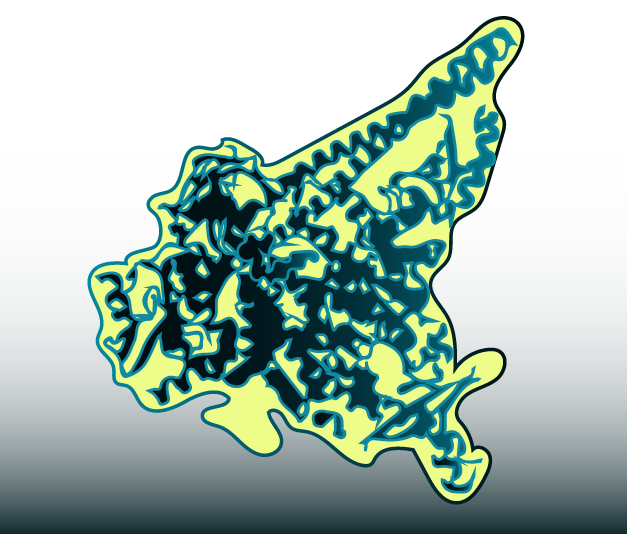
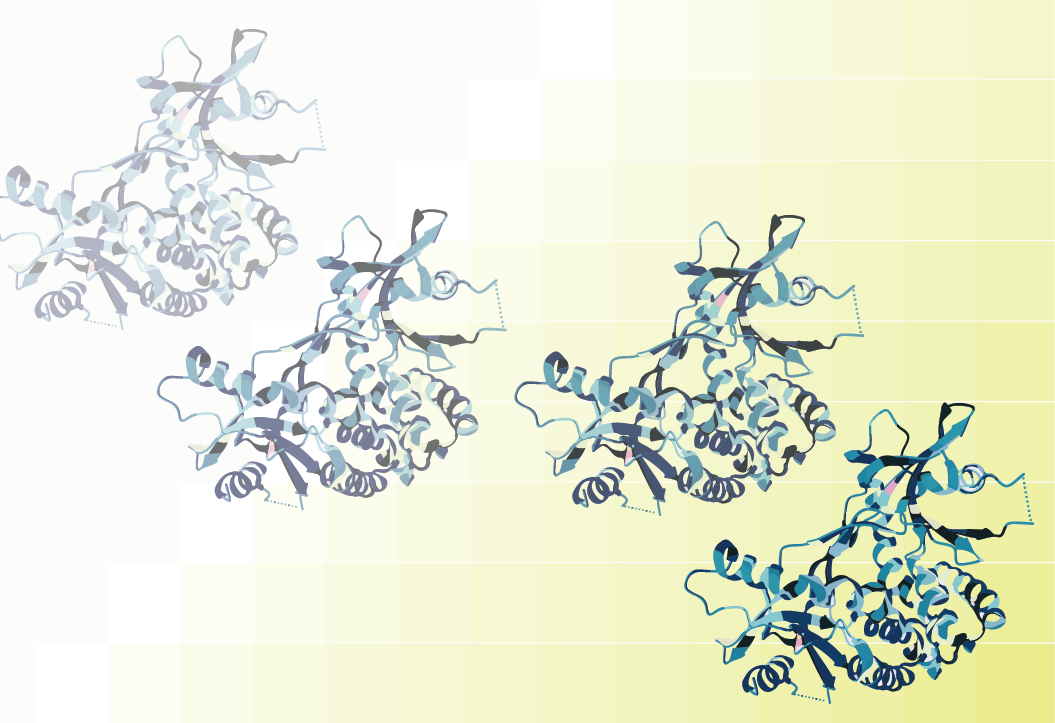
Figure 1: illustration of model PI3Kα with its regulator subunit (both in dark blue) and the approximate binding site for the novel activator UCL-TRO-1938.
Confirmed hits were counter-screened for both PI3K activity and binding and then validated in an engineered human cell line. Their screen identified a novel compound, dubbed UCL-TRO-1938 (1938) that acts as an allosteric PI3K activator. Crucially, 1938 appeared specific for a distinct Class 1 PI3K isoform, PI3Kα, and showed no activity towards Class II or III PI3Ks. Phosphoproteomics data also showed that the compound appears to activate the classical PI3K pathway, although some phosphorylation sites identified in the data set had not been previously attributed to PI3K signaling. 1938 also performed favorably with two models of tissue protection and regeneration using perfused hearts and sciatic nerves from rats. This aligns with previous work showing that activation of PI3Kα is a positive effector of axon regeneration and is necessary for cardioprotection. In total, the current data points to 1938 being a novel, effective activator of PI3Kα that will enable larger pharmacological investigation of PI3Kα signaling for therapeutic development.
Read the full article here:
A small-molecule PI3Kα activator for cardioprotection and neuroregeneration
Nature 618:159-168 (2023)
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences