Hyaluronic acid (HA) is a fascinating bioactive polymer involved in various cellular signaling pathways. It has been extensively studied in scientific research and has also gained public attention in recent years, especially in the cosmetic industry for its hydrating and skin-enhancing properties.
The homeostasis of HA is tightly regulated by two key enzymes: hyaluronic acid synthase (HAS) and hyaluronidase (HYAL). There are three isoforms of HAS, each responsible for synthesizing HA of different molecular weight. Similarly, there are five functional isoforms of hyaluronidase—HYAL1 through HYAL4, and SPAM1—which degrade HA into fragments of varying sizes. These HA fragments can trigger a range of physiological and pathological responses. In fact, elevated levels of hyaluronidase have been associated with cancer progression, chronic inflammation, skin aging, neurodegenerative disorders, and infections.
Clinically, hyaluronidase has been widely used as co-administered drug to enhance drug delivery by facilitating the penetration of the therapeutic agents through the extracellular matrix. Despite its therapeutic potential, no FDA-approved hyaluronidase inhibitors currently exist. Inhibition of hyaluronidase could offer therapeutic benefits in limiting cancer invasiveness, modulating chronic inflammation, neutralizing venom, and extending the longevity of cosmetic fillers. However, targeting this enzyme poses challenges due to its broad physiological roles and potential off-target effects, emphasizing the need for thorough characterization during inhibitor development.
A key step in identifying hyaluronidase inhibitors is screening compounds for their effect on hyaluronidase activity. Traditional turbidity assays, such as the one offered by Bioassay Systems, measure hyaluronidase activity by monitoring the reduction in solution turbidity, which is effective for high-throughput screening of compound libraries. For in-depth inhibitor characterization, assays that directly quantify substrate consumption or product formation offer a more comprehensive assessment of the inhibitor effects. Echelon’s Hyaluronidase Activity ELISA addresses this need by directly measuring the hyaluronidase degradation of hyaluronic acid (HA), providing a precise and quantitative result in microgram of HA.
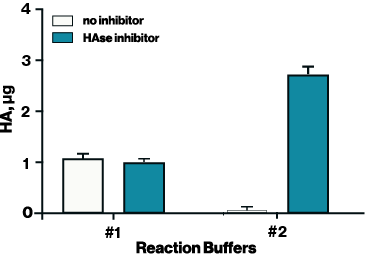
Figure 1. Hyaluronidase activity was tested with two different reaction buffers with and without a HAse inhibitor to optimize conditions for inhibition. HAse inhibitor (Echelon B-0601) was diluted in DMSO and applied at 200 uM.
To adapt the Hyaluronidase Activity ELISA for inhibitor screening, it is essential to use a reaction buffer that supports both enzyme activity and inhibitor compatibility. For example, the standard reaction buffer provided with the ELISA (Buffer #1 in Figure 1) works well for measuring hyaluronidase activity but was incompatible with the HAse Inhibitor (EBI Cat# B-0601). In contrast, Buffer #2— 50 mM ammonium formate at pH 5.2—supported both hyaluronidase activity and inhibitor effectiveness, allowing the determination of IC₅₀ values for two known hyaluronidase inhibitors: HAse Inhibitor (2.357 µM) and delphinidin (30.22 µM) (Figure 2).
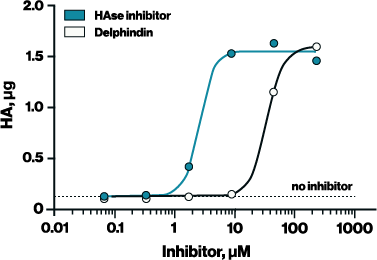
Figure 2. A recently identified hyaluronidase inhibitor, Delphindin, was assessed for activity using the optimized conditions from Figure 1. HAse inhibitors were diluted in DMSO and applied at 200 uM in ammonium formate buffer for 1 hour at 37C.
In summary, hyaluronidase represents a compelling therapeutic target across oncology, inflammation, and tissue remodeling. While its inhibition holds significant therapeutic promise, continued research is needed to develop selective, effective hyaluronidase inhibitors with minimal off-target effects.
Ready to start your own hyaluronidase study? Check out our full hyaluronic acid product portfolio.
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences