Alzheimer’s disease (AD) has long been characterized by the presence of plaques in neuronal tissue composed primarily of amyloid beta. More recently, there has been an increasing effort to understand the contributions of non-neuronal subtypes to AD, specifically immune cells such as microglia. Current evidence has shown that these cells are active players in the disease process and some genomic analyses have shown that innate immune activity is involved in late-onset Alzheimer’s disease (LOAD). However, the precise molecular players in neuroimmune cells have yet to be defined.
Several genome-wide association studies (GWAS) have identified mutations within the INPP5D gene that are associated with LOAD. INPP5D, aka SHIP1, is known to play a role in lipid metabolism within immune cells through its action as a phosphatase on PI(3,4,5)P3 and its activity has been associated with proper functioning of macrophages and microglia. INPP5D’s role in LOAD though, remains unclear. While the GWAS analyses point to specific mutations that are predicted to affect INPP5D activity, studies of microglial expression of INPP5D in AD mice have been conflicting.
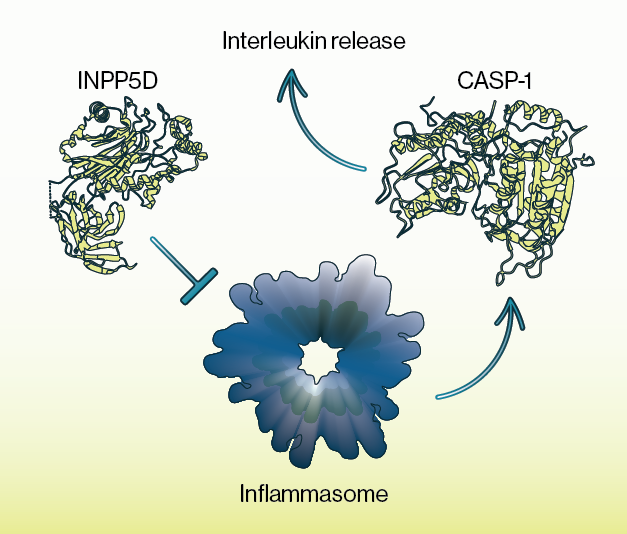
In the current study, Chou and colleagues provide evidence that INPP5D expression is highest in the brain within microglial cells and that its expression is elevated in AD. Using a combination of immunocytochemistry and western blotting, they show that INPP5D protein is elevated at microglial plaque sites but depleted within aqueous fractions of the cell. Furthermore, these alterations in INPP5D protein appeared to be isoform specific as INPP5D found around plaque sites did not contain the active phosphatase domain. Subsequent experiments using small molecule inhibitors and genetic reduction via CRISPR demonstrated that INPP5D acts as a regulator of the inflammasome, a large multi-protein oligomer that is part of innate immune sensing. Follow-up tests using a co-culture system of microglia and neurons showed that this impairment of INPP5D activity led to wide ranging alterations in gene expression in neurons, including some known AD risk genes.
The current data provide a potential link between the emerging involvement of immune cells and inflammatory pathways in AD with more canonical markers of the disease. While it remains to be seen if pathways can be exploited to treat disease, the authors propose two plausible routes that could have therapeutic significance: inhibition of inflammasome activity or targeting INPP5D. The apparent isoform specific nature of INPP5D’s involvement suggests that there may be multiple avenues to effectively target this gene and its protein.
Read the full article here:
INPP5D regulates inflammasome activation in human microglia
Nature Communications (2023)