The cGAS/STING pathway is a critical part of the innate immune system that detects cytosolic DNA and promotes inflammatory responses. While this pathway is primarily triggered by foreign DNA from viruses and bacteria, it can also recognize self-DNA that arises due to autoinflammation or cellular damage as seen in cancerous cells. Some RNA viruses may also trigger STING activation through various mechanisms, including lipid membrane alterations, though viral inactivation of STING has also been observed. Given its involvement in viral immune responses, autoimmunity, and tumorigenicity, there has been great interest in understanding the full scope of STING regulation.
STING activation is dependent on ligand binding, primarily in the form of cyclic dinucletotides (CDNs) that are produced from certain pathogens or by cyclic GMP-AMP synthase (cGAS) in mammals. cGAS catalyzes the formation of cyclic GMP-AMP (cGAMP) when it detects and binds cytosolic DNA. cGAMP can then activate STING which initiates its translocation from the ER to the Golgi and subsequent activation of immune transcription factors, though the process of translocation is not well understood.
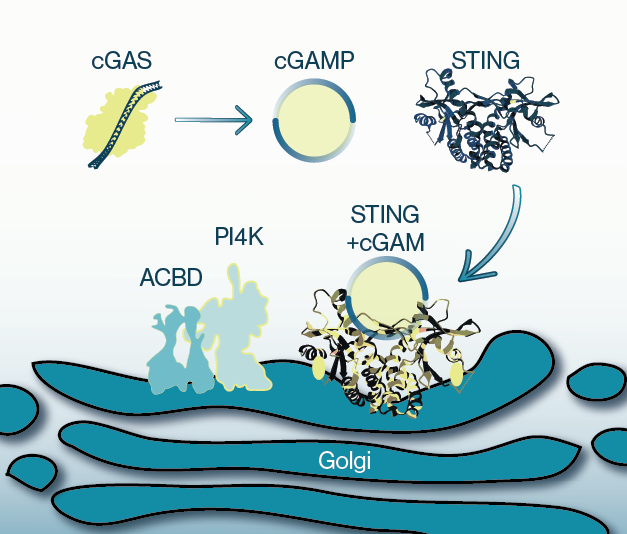
In the current study, a genome-wide CRISPR screen was conducted to identify modulators of STING activity. One of the top hits from the screen was a Golgi-resident protein called ACBD3 which is known to facilitate ER-to-Golgi vesicle trafficking as well as recruitment of signaling proteins to the Golgi. One such protein is the enzyme PI4-kinase (PI4KB), which synthesizes the lipid PI4P. PI4P is known to recruit proteins to the membrane withing the ER-Golgi network and increasing local membrane concentrations of PI4P can drive activity of protein complexes. Here, the authors show that ACBD3 facilitates localization and activity of STING on Golgi membranes by driving activity of PI4KB and subsequent PI4P synthesis. Inhibition of PI4KB reduced STING activity and mutation of a PI4P binding site in STING abrogated its activation. Conversely, targeting STING to sites enriched with PI4P enhanced its activity. Taken together, the current data suggest a new route by which ACBD3-PI4K-PI4P signaling can be manipulated to therapeutically utilize STING activity.
Read the full article here:
The activation of the adaptor protein STING depends on its interactions with the phospholipid PI4P
Science Signaling (2024)