Testing is one critical tool to control the SARS-CoV-2 pandemic and other viral outbreaks, saving lives and keeping the economy afloat. Viral testing and antibody testing are the two main testing methods, but have different uses. Viral testing by PCR is the recommended diagnosis method by the CDC for determining a current infection. Conversely, antibody testing from blood serum, a serology test, is an alternative clinical surveillance and research tool to understand the transmission dynamics of the virus. Once a patient is infected with SARS-CoV-2 virus, SARS-CoV-2 specific antibodies will present in the patient’s blood after one to three weeks, whether symptoms show up or not. Thus, having reliable and flexible serology tests is critical for tracking viral spread amongst citizens that may not have had a PCR test for a current infection.
Serologic Assays
Serologic tests look for antibodies in your blood and can involve a number of laboratory techniques. Different types of serologic tests are used for various diagnoses and many types of serologic assays are available in the market. These include laboratory-based immunoassays such as enzyme-linked immunosorbent assays (ELISAs), chemiluminescent microparticle immunoassays (CMIAs), and on-site rapid diagnostic tests (point-of-care tests). However, they all commonly involve proteins and antigens generated by the immune system. For tracking SARS-CoV2 infections and COVID-19 cases, recombinant forms of the SARS-CoV-2 S protein and N protein are the common antigens for development of antibody tests. These antigens are not without their drawbacks as they are relatively high cost, have batch-to-batch variation, and can exhibit cross-reactivity with other antibodies created by common coronaviruses. Peptide antigens can overcome these hurdles.
A recent study from Lisa F.P. Ng and colleagues at the National University of Singapore reported four specific peptides from the S and N proteins of SARS-CoV-2 that appeared to predominate in human serum.1 These peptides, listed below and highlighted in Figure 1, displayed high degrees of sensitivity and specificity in detecting SARS-CoV-2 antibodies.
Spike (S) glycoprotein Sequence
S14P5 (553-570) TESNKKFLPFQQFGRDIA (Echelon #732-23)
S20P2 (769-786) GIAVEQDKNTQEVFAQVK
S21P2 (809-826) PSKPSKRSFIEDLLFNKV (Echelon #732-24)
Nucleocapsid (N) protein Sequence
N4P5 (153-170) NNAAIVLQLPQGTTLPKG (Echelon #732-21)
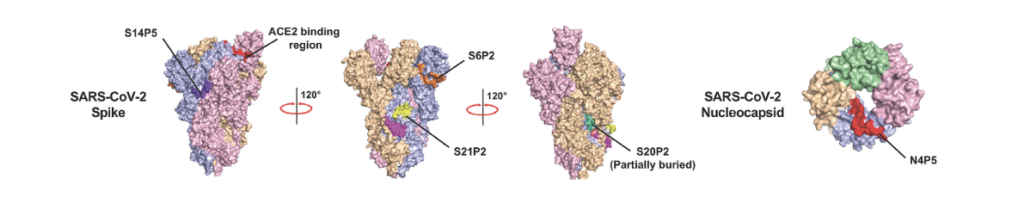
Figure 1: Schematic of the structure of the SARS-CoV-2 spike (S) protein with locations of immune epitopes highlighted. Adapted from Amrun et al. 2020.
Among the four epitopes, N4P5 achieved the highest level of specificity (100%) and sensitivity (>96%) against SARS-CoV-2. In addition, combining two or more of the epitopes can provide great sensitivity and specificity. IgG levels against those epitopes in COVID-19 patients were also associated with disease severity. Similar responses of S proteins were also found. High IgG levels against the N4P5 epitope correlated with lymphopenia in COVID-19 patients. The potential mutation rates of the four peptides are low. The authors also reported that antibodies against S14P5 and S21P2 can neutralize SARS-CoV-2.2
Clinical and Therapeutic Outlook
In addition to the reported sensitivity and specificity of these peptides for SARS-CoV-2, epitope-specific immune responses to these peptides appear to correlate with disease severity. This observation would add another valuable component to diagnostic assays as simple positive-negative tests could double as indicators of current or past disease severity. This has implications both for how therapeutic options are deployed but also for future community tracing.
Currently, mutation rates in these epitopes appear to be low, however this may change as SARS-CoV-2 lingers in the global population. As with the new mRNA vaccines, an added benefit of using peptide based assays is that peptide synthesis is highly flexible. This means that new peptides can be synthesized to match changing epitopes in the event that new variants of SARS-CoV-2 arise and become predominant. Implementation of peptide-based assays is also generally more cost-effective than antibody based assays, which would lower barriers to effective testing in developing countries affected by SARS-CoV-2 and other novel viruses.
Find more literature, reagents, and assays related to COVID-19 below.
References
1. Siti Naqiah Amrun, et al. (2020) Linear B-cell epitopes in the spike and nucleocapsid proteins as markers of SARS-CoV-2 exposure and disease severity. EBioMedicine, (58),102911
2. Poh, C.M., et al. (2020) Two linear epitopes on the SARS-CoV-2 spike protein that elicit neutralising antibodies in COVID-19 patients. Nat. Commun. 11, 2806.
0.2
/ 0.3
Related Articles
Stay informed with our informative blog posts.
0.3
/ 0.3
Get in Touch
If you have any questions or would like to learn more about our services, feel free to reach out to us. We’re here to help!
Biosciences